Recommendation Info About How To Find Out Protons Neutrons And Electrons
Repeat for each element in the molecule, then sum together all the.
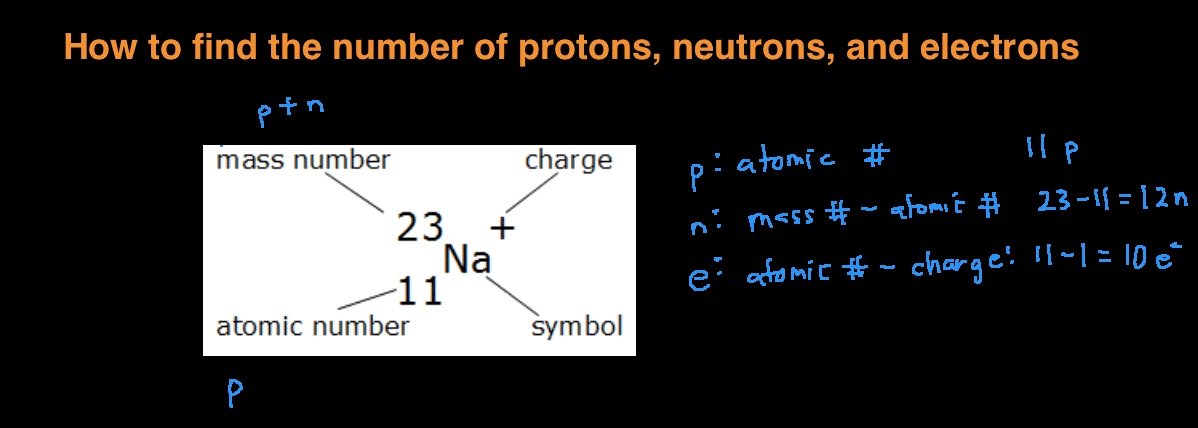
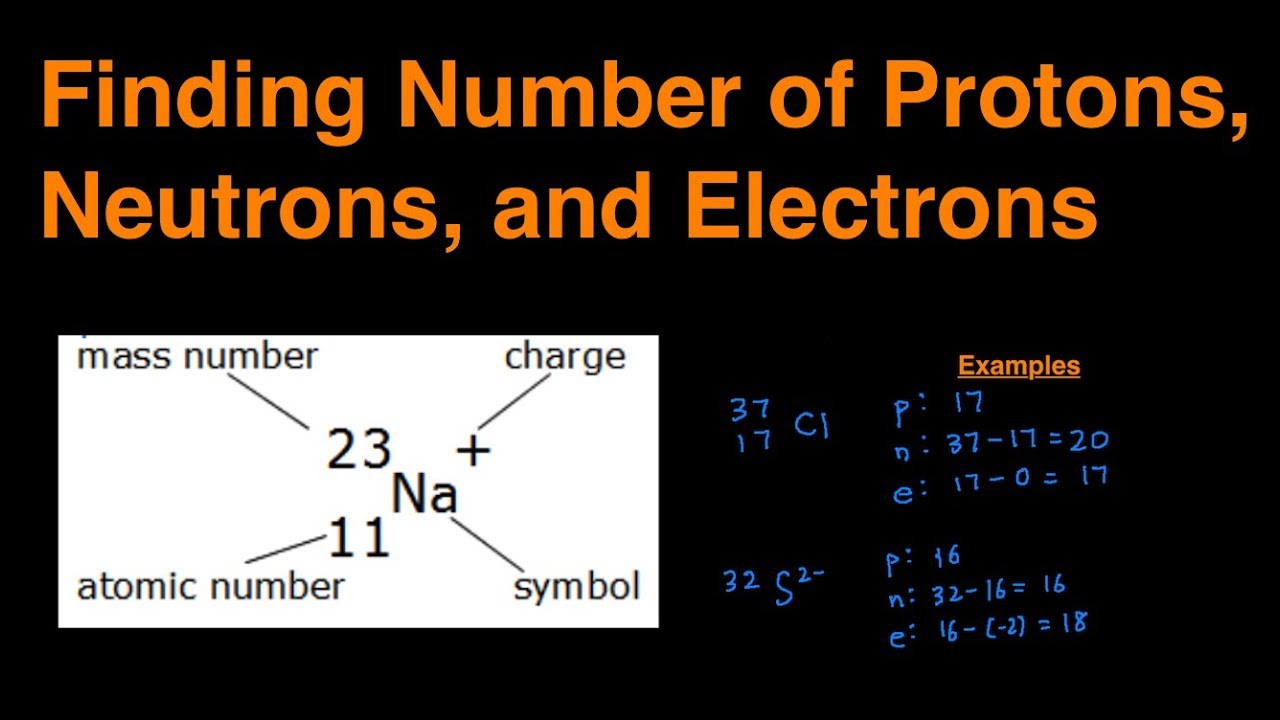
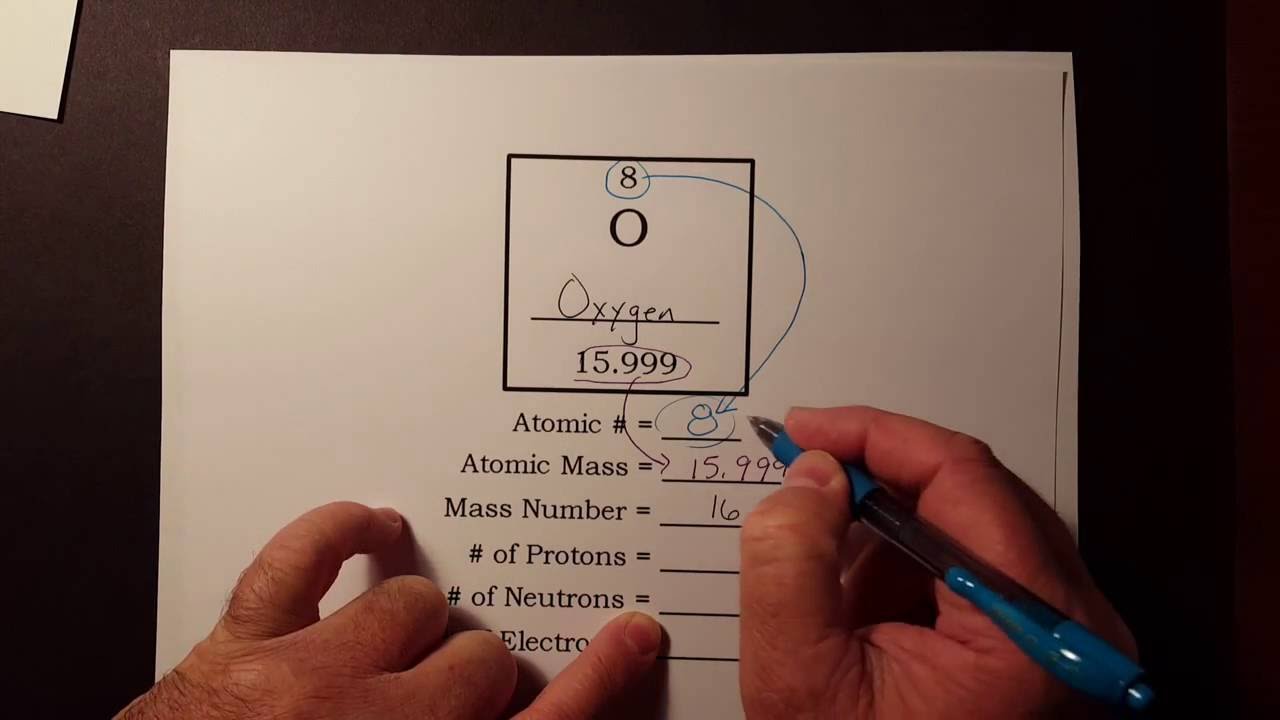
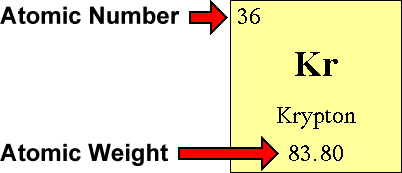
How to find out protons neutrons and electrons. How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? You can use these numbers to calculate the number of. The number of neutrons can be found by subtracting the atomic number from its atomic mass.
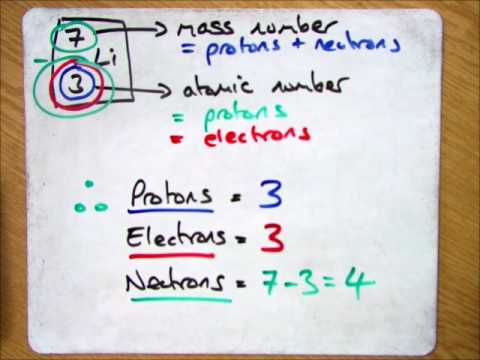
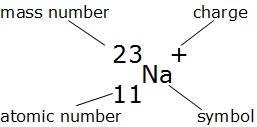
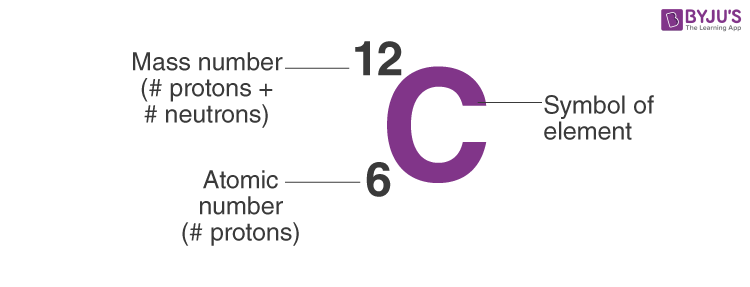
When we write the symbol for an atom, we can place its mass number at the top left and its atomic number at the bottom left. The number of protons can be found by knowing the atomic number of that atom. Finding the protons, neutrons and electrons in scandium.
It also explains the differe. Atomic mass (a) = nucleus mass = total mass of protons and neutrons (p + n) again, the mass of each proton and neutron is about 1amu. Bohr model drawing (25 points) identify the element symbol of.
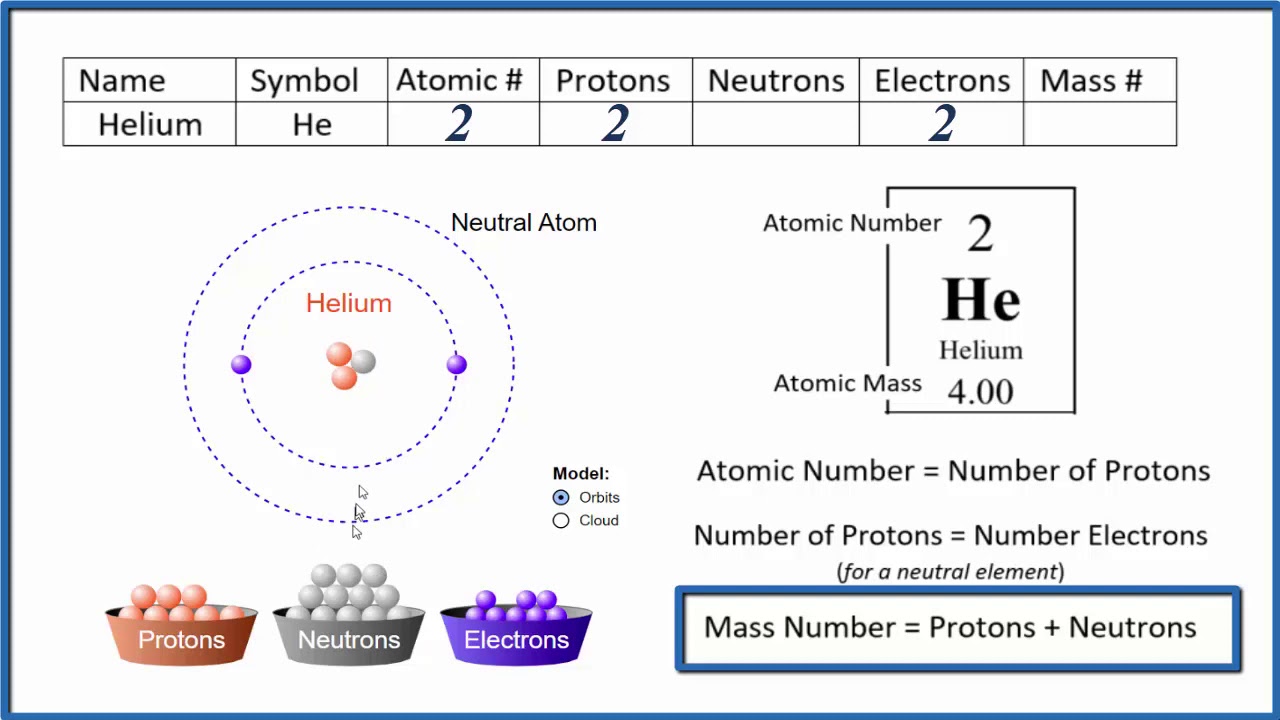
Repeat for each element in the molecule, then sum together all the products to get the. Number of electrons in potassium = atomic number of potassium = 19. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion.
The periodic desk is a catalog that organizes elements by their atomic structure. For a neutral atom, the number. The number of neutrons can be found by subtracting the atomic number from its atomic mass.
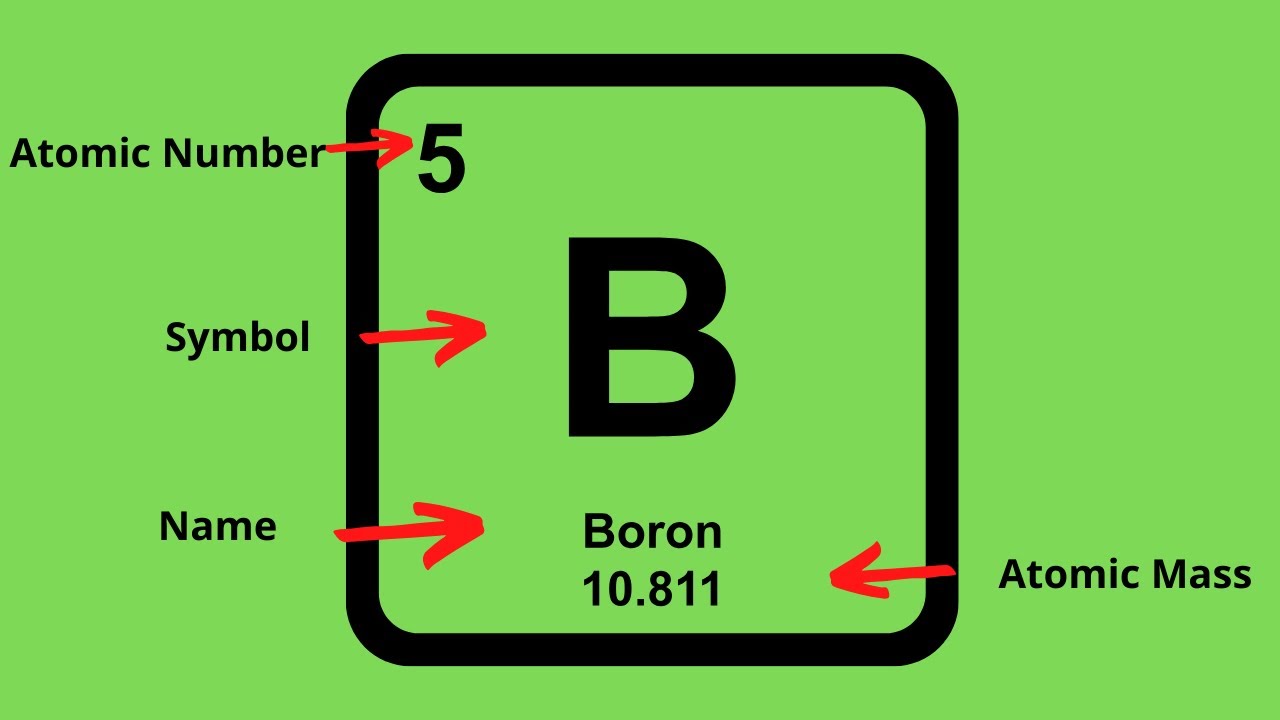
Off protons = atomic number. Multiply the element’s atomic number by the number of atoms of this kind in the molecule (see step 1). The elements in the periodic table are arranged according to their atomic number.